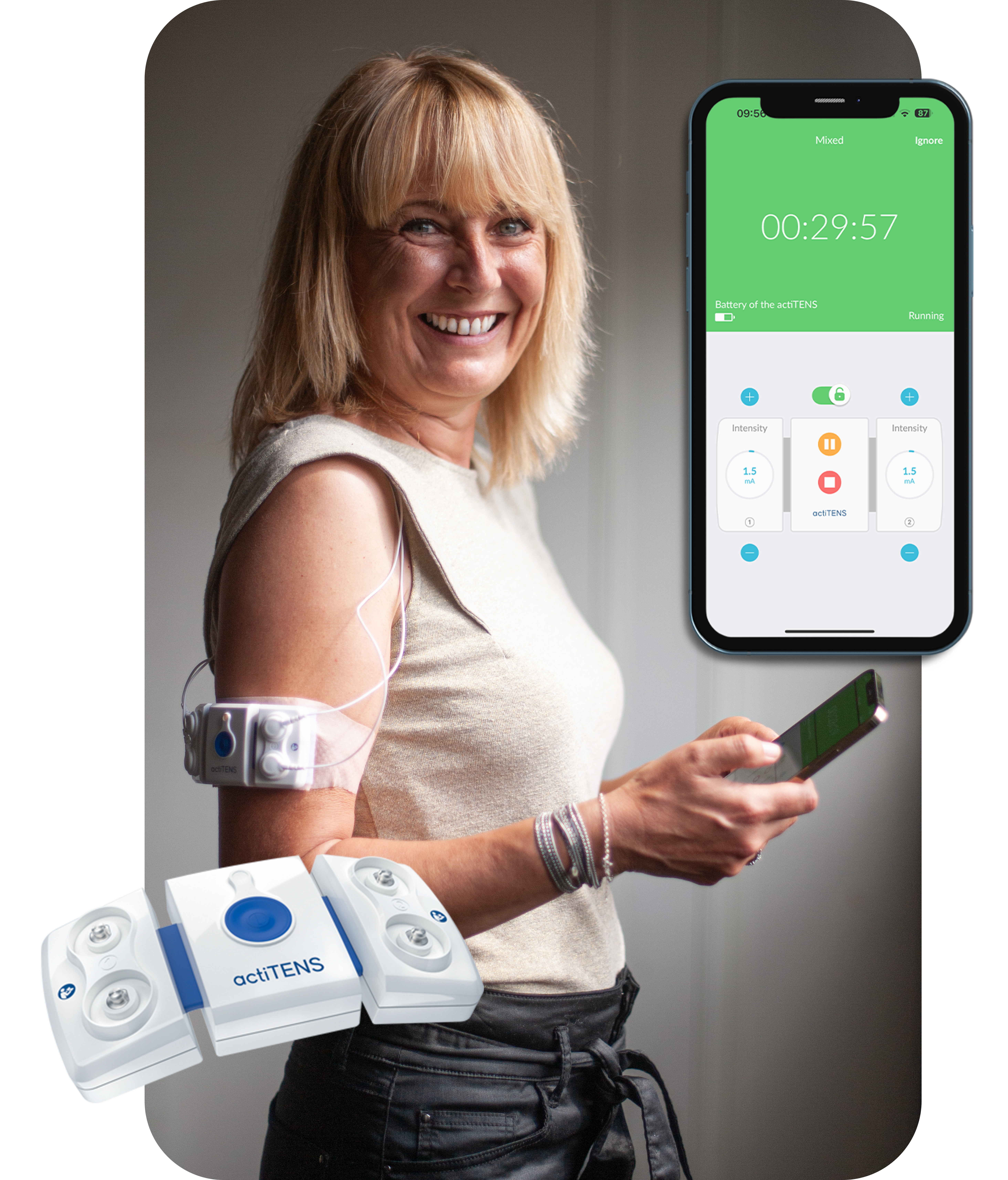
- About actiTENS®
A hope for pain sufferers!
Versatile TENS and EMS technology
actiTENS is a mobile and versatile solution that dynamically adapts to different physical contours and pathologies, with a wide range of fastening accessories and electrodes including multisite and low back electrodes offering a personalized and effective treatment experience for pain relief.
stimulation sessions
Trusted patient
Reviews
Connected
actiTENS is controlled by our mobile application, enabling people to create a patient account and benefit from patient follow-up.
Ergonomic
actiTENS is a device designed to adapt perfectly to different areas of the body and their contours.
Discreet
The discreet design of actiTENS allows patients to follow their therapy program at any time.
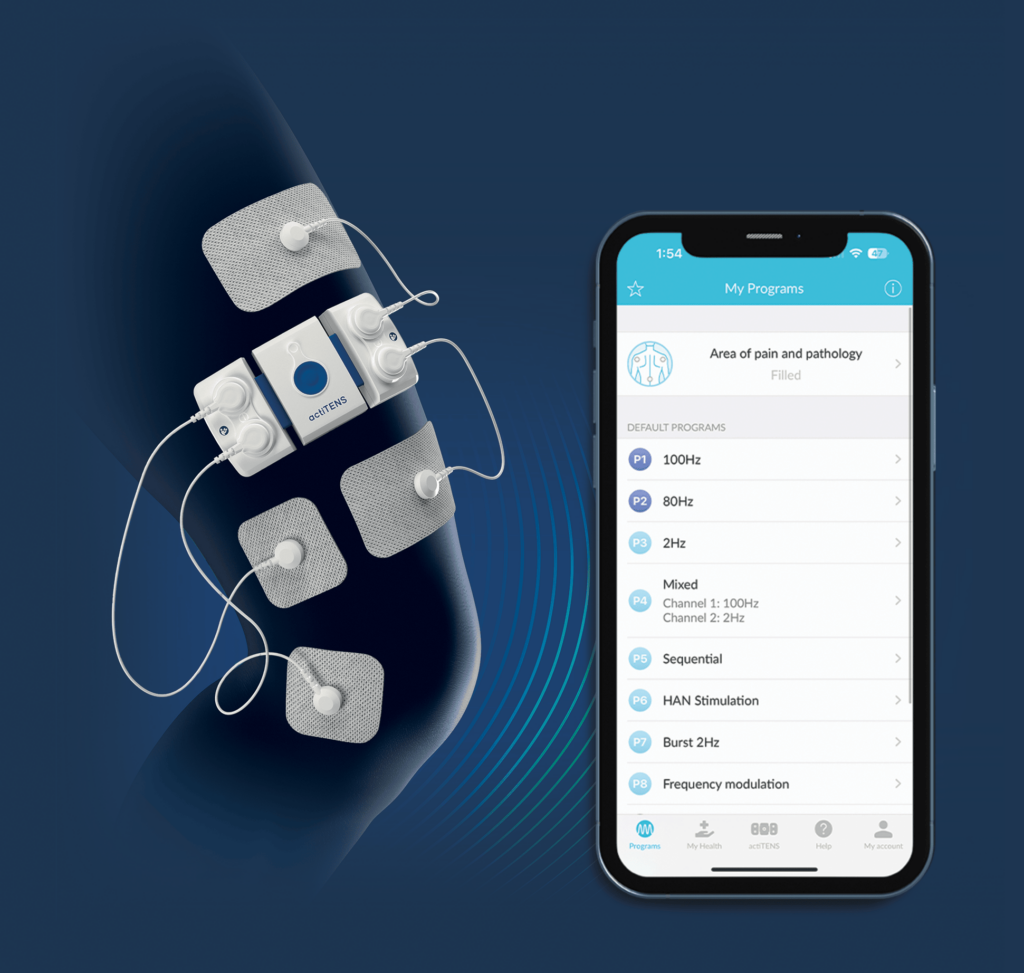
- actiTENS® application
Pain relief in your hands
With our interactive smartphone app wirelessy activate treatment, store personalized treatment data and see results of your therapy!
Patient account
Programs
Remote control
Pain impact
- actiTENS - Efficient device
A new wearable transcutaneous electrical nerve stimulation device (actiTENS) is more efficient and better tolerated than weak opioids in the treatment of knee osteoarthritis pain⁵.
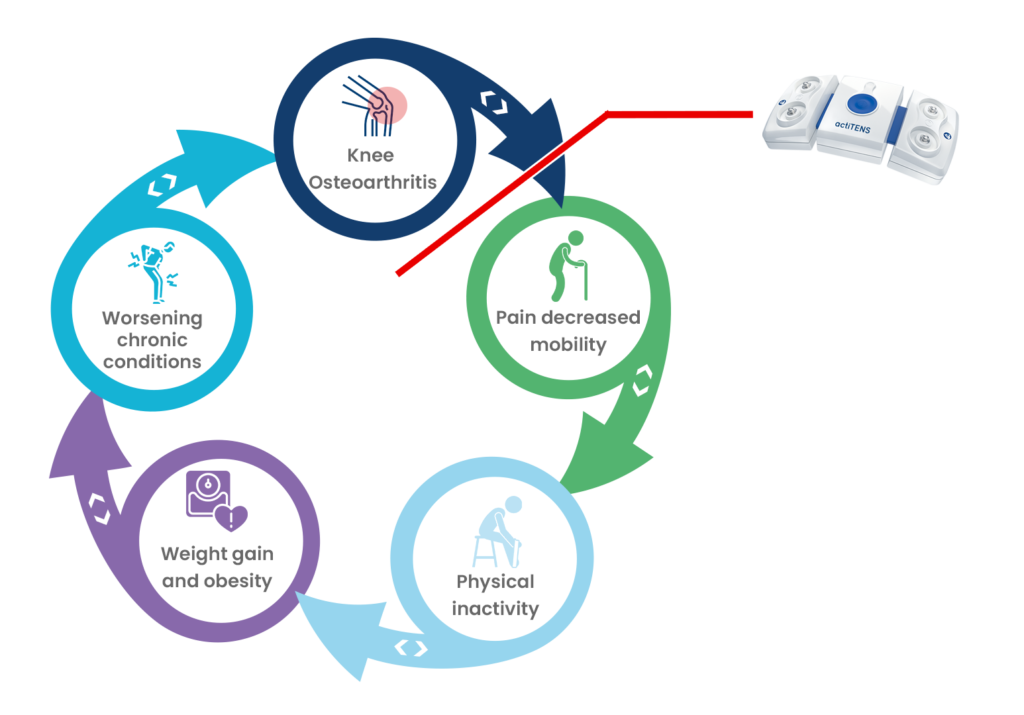
The debilitating effects of KOA
Osteoarthritis of the knee restricts mobility, potentially causing reduced independence, weight gain, and worsening of chronic conditions, highlighting the importance of breaking this cycle to slow down the disease.
- KOA - CLINICAL STUDY
actiTENS has proven to be more effective than Level 2 analgesics.
A clinical study conducted with 55 patients utilizing actiTENS for knee osteoarthritis pain management, compared to a second group of 55 patients consuming Level 2 analgesics for the same condition, revealed the following outcomes⁵.
2.06 points
Mean reduction in pain intensity
Patients using actiTENS show a significant pain reduction of 2.06 points after 3 months, surpassing the efficacy of weak opioids.
Current activities
After 3 months of use, 84% of patients reported improved daily functioning based on responses collected through the WOMAC® questionnaire⁵.


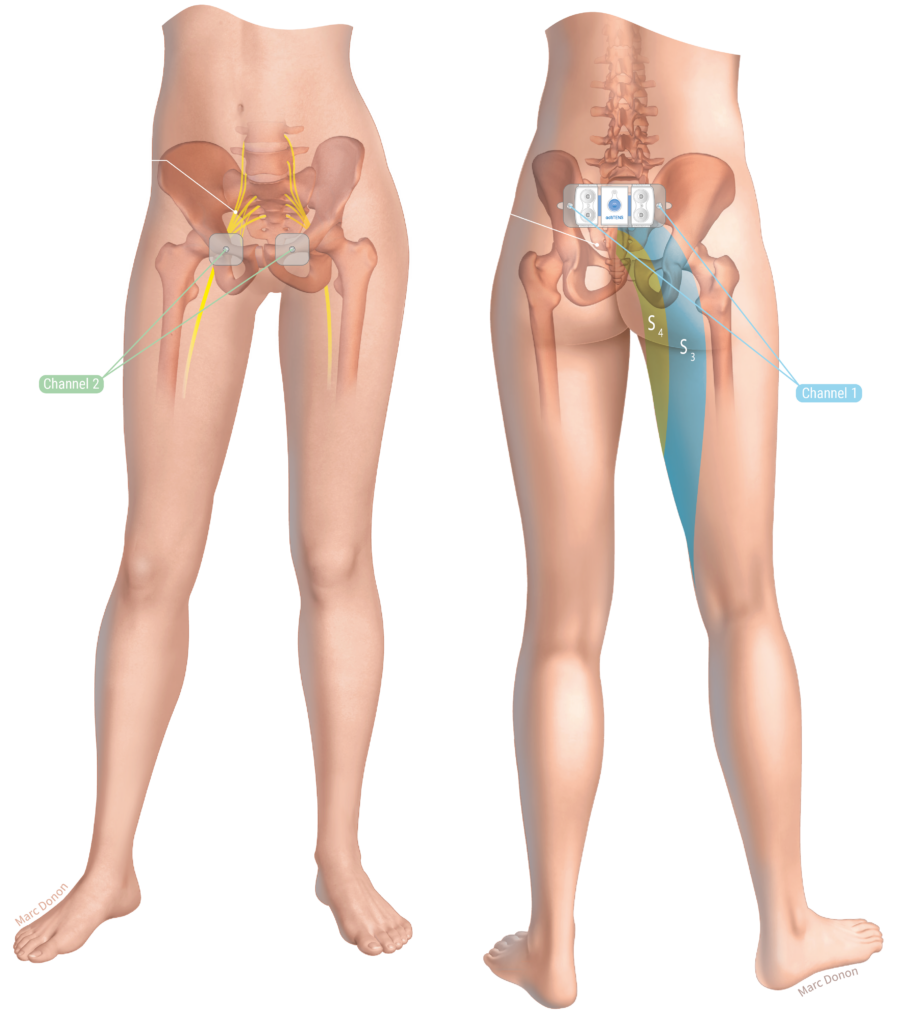
actiTENS® in endometriosis pain management
Endometriosis affects roughly 10% (190 million) of reproductive age women and girls globally⁶.
the most frequently reported symptoms of endometriosis are pelvic pain (92.5%), dyspareunia (80%) and heavy bleeding (75%)⁷.
Endometriosis has significant social, public health and economic implications. It can decrease quality of life due to severe pain, fatigue, depression, anxiety and infertility⁸.
actiTENS is an option for the management of endometriosis-induced pain
actiTENS offers TENS and EMS mode stimulation programs designed to alleviate endometriosis pain and to provide targeted relief
- ENDOMETRIOSIS - INAPP SURVEY
actiTENS could be a relevant non-pharmacological therapeutic option for the management of pain in endometriosis.
Inapp survey conducted from 03/17 to 04/10/23 among 127 patients utilizing actiTENS for managing endometriosis pain has demonstrated the following outcomes: improvements in pain levels, quality of life, activity limitations, and reduced medication consumption¹.
of patients using actiTENS reported an improvement in their pain
of patients using actiTENS reported an improvement in their quality of life
of patients, actiTENS results in an improvement in activity limitations
of patients using actiTENS reported a reduction in drug consumption
Control of actiTENS via a mobile application enables us to carry out real-life surveys with patients to assess the device's effectiveness.
actiTENS vs competitors, from a patient's point of view
InApp survey conducted from 31/05 to 26/06/23 among 68 patients new users of actiTENS suffering from various pathologies and having previously used another TENS⁹.
of patients who had previously used other TENS devices find actiTENS offers greater discretion.
of patients using actiTENS consider it to be easier to use than other TENS already used.
actiTENS offers improved use of TENS therapy at work, outdoors and in daily activities for 82% of its users.
of patients report improved efficacy of actiTENS in reducing pain compared to other TENS used.
Control of actiTENS via a mobile application enables us to carry out real-life surveys with patients to assess the device's effectiveness.

- Mission & Contact
Our mission is
To develop and offer sustainable, clinically-supported drug-free therapeutic solutions to improve quality of life and support pain sufferers worldwide.
Our location
137 rue mayoussard,
38430 Moirans FRANCE
Contact our support
Mobile : +1 (865) 748-8562
Phone : +33 (0) 4 76 37 17 58
E-mail : contactUS@sublimed-technologies.com

This product is truly revolutionary, I don’t need to shoot up with painkillers anymore... The application is very easy to use and the after-sales service is very efficient. I highly recommend this product!

Super device and super app, which allow me both to combat my chronic pain and to passively strengthen certain muscles. The equipment is top-quality and modern, and the device can be controlled via the app. Customer service is also top-notch. I'm very satisfied.

Super device, I'm extremely satisfied with it, I use it every day for at least 3 hours, my neuropathic post-chemotherapy pain has halved. Thanks to this device, I've considerably reduced my consumption of painkillers. What's more, the helpline is really top-notch, very responsive and guarantees user satisfaction. I couldn't do without it, and I highly recommend it.

I've been using the actiTENS since September 2018. Almost 6 years of use without a single breakdown, which is rare reliability these days. It's a real marvel and has quickly become indispensable. The application is easy to use, the equipment extremely efficient, and, above all, very discreet. With numerous programs and remarkable autonomy, I highly recommend it to people who need to return to work and want to continue their daily care routine.

Very intuitive to use. actiTENS electrodes are highly effective and, unlike others, can be used for longer periods of time. Very light and discreet, for use anywhere. Well done! I recommend this treatment device.
- Testimonial
What Our Patients Said
We have more than 2257 reviews on our store, join us in our commitment to a pain-free world !
Sources
1– The InApp survey conducted from 03/17 to 04/10/23 among 127 patients using actiTENS for the management of endometriosis pain.
2– The inApp survey conducted from 10/21 to 11/25/21 among 183 patients using actiTENS for the relief of various types of pain
3– https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5319385/
4– https://www.medpagetoday.com/resource-centers/clinical-challenges-knee-osteoarthritis
5– https://www.oarsijournal.com/article/S1063-4584(21)00599-9/fulltext
6– https://www.who.int/news-room/fact-sheets/detail/endometriosis
7– https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10241351/
8– https://www.who.int/news-room/fact-sheets/detail/endometriosis
9– InApp survey conducted from 31/05 to 26/06/23 among 68 patients new users of actiTENS suffering from various pathologies and having previously used another TENS.
Download Our App
Explore an innovative approach to pain relief by downloading the actiTENS app now!
SAS Sublimed 813 959 012 00010 – Réf : SBM1AM021_US – V1 – 2024
© Copyright – All rights reserved.